COVID-19 and Vaccines
Sadly, we have reached a new milestone. More than 500,000 people have died from COVID-19 in the U.S. thus far. But there is new hope. On Dec 11, 2020, The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the Pfizer vaccine against COVID-19, and exactly a week later, on Dec 18, 2020, FDA issued an EUA for the Moderna vaccine. EUA is given during a public health emergency, when the scientific evidence available shows that the product may be effective and that the known and potential benefits of the product outweigh the known and potential risks of the product. Often, in due time, once more data is gathered, full FDA approval is obtained. It is important to know, however, that the FDA’s review of the vaccines for the EUA was thorough and rigorous, with no steps skipped to ensure the safety of these vaccines. Thousands of people participated in these clinical trials and millions have safely taken these vaccines to this point.
Both these vaccines are mRNA vaccines. They have a synthetic genetic material called mRNA that instructs your cells to make a unique piece of the COVID-19 virus called the “spike protein”. This then causes your body’s immune system to produce antibodies against the “spike protein”, hence protecting you from the real virus if you are ever exposed. It is very important to note that you cannot develop the actual COVID-19 disease from the vaccine.
Currently, the Moderna vaccine is approved for people 18 years and older and the Pfizer vaccine for people 16 years and older. These vaccines are given to people in two doses, 21 days apart for Pfizer or 28 days apart for Moderna. The effectiveness of the vaccines has only been studied after two doses, so it is important you get both doses. After both doses, people can be protected from severe COVID-19 disease up to 95%. The CDC allows for a 4-day grace period for timing of the second dose, however, if your second dose is for some reason given later than this, you do not need to restart the vaccine. The CDC has recommended that people get the same version of vaccine for both doses, because that is what the data in the clinical trials were based on. They have indicated, however, that in exceptional circumstances, if the same version is not available, a person can take a different version as second dose.
The most commonly reported side effects are soreness at the site of the injection as well as flu-like symptoms, including fatigue, body aches, chills or fevers after the vaccine. These self-limiting side effects tell us that the body is building protection against the virus. To date, people who have had allergic reactions to the vaccine have all recovered quickly. This risk of allergic reaction is very small and is similar to the risk of allergic reaction associated with any other medication or vaccine. At this time, there are no known reactions or interactions between oral medications and the vaccines, so it is safe for you to get it even if you are on any medications.
While we know the vaccine prevents you from getting severe COVID-19 disease, we are not sure yet to what extent it prevents the spreading of the virus that causes COVID-19. For this reason, even after you receive the vaccine, you should continue social distancing, wearing masks in public and following other CDC guidelines to reduce risk of transmission at this time. These restrictions will likely ease up in future as more and more people get vaccinated. The vaccine will provide you and your family an added layer of protection against this horrible COVID-19 disease and also will help us become one step closer to achieving herd immunity and conquering this pandemic.
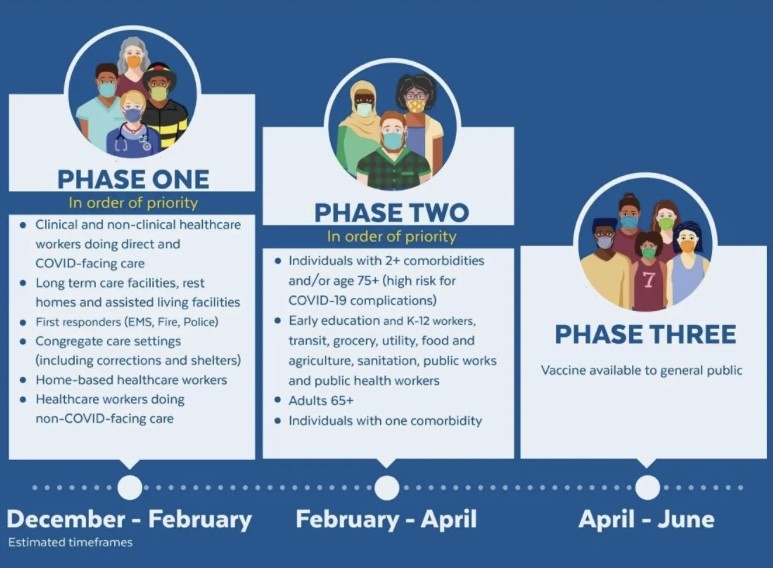
The Massachusetts Department of Health has developed a plan to vaccinate everyone in the state who wants to be vaccinated. Doing so will take several months. Below is a chart explaining the phases in which vaccine rollout is happening. We are currently in phase II.
Vaccines are being offered to eligible people at several different types of locations:
-
Mass vaccination sites such as at Gillette Stadium, Fenway Park, the DoubleTree in Danvers, the Eastfield Mall in Springfield, the Natick Mall, and the former Circuit City in Dartmouth
-
General vaccination sites such as health care locations, pharmacies and grocery stores
-
Local vaccination sites which may be open only to residents of select and towns. Please get information about when and where you can get yours and get it!
https://www.mass.gov/info-details/massachusetts-covid-19-vaccination-phases
Social media can easily and quickly spread information, including false information, so it is important that you go to reliable sources like the CDC, FDA and your doctor for accurate information. Here are some useful links:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/keythingstoknow.html
Finally, multiple variants of the virus that causes COVID-19 are circulating globally. Specifically, variants from the UK, South Africa and Brazil have gained attention. These variants seem to spread more easily and quickly than other variants, which may lead to more cases of COVID-19. So far, studies suggest that antibodies generated through vaccination with currently authorized vaccines recognize these variants but the protection may be less. There may soon be vaccine booster shots available to address these new variants.
–Dr. Anasuya Gunturi
